|
|
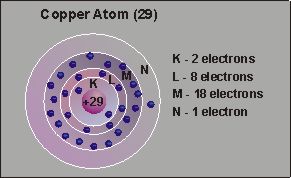
SemiconductorsPart 2: ConductorsElectrical conductors are elements with only one to three electrons in the outer shell of each atom. The best conductors - copper, gold and silver - have only one electron in the outer shell, mercury has two and aluminium has three. Because the outer shell is not filled, the electrons tend to move easily from one atom to another within the element. Because the movement is random, no electrical change results in the absence of an electrical force. However, when a voltage is applied to a conductor, the negative electrons will move rapidly towards the positive voltage. This is known as current flow.
|