|

|
Semiconductors
Part
4: Silicon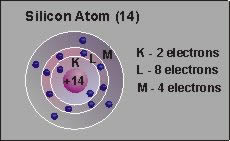 and Germanium
and Germanium
Semiconductors
are a unique group of elements which have four valence electrons.
The most common examples are silicon and germanium. These elements
are poor conductors at low temperatures, but their conductivity
increases as temperature rises. Silicon is generally preferred for
manufacturing semiconductor devices because it is not as sensitive
to temperature variations. Pure silicon does not exist naturally,
but is found as an oxide or silicate in sand, and numerous minerals.
Sand is used in the manufacture of glass which is an excellent insulator.
When pure silicon is produced, it forms a crystal in which the four
valence electrons are shared by adjacent atoms, forming what is
known as a covalent bond. 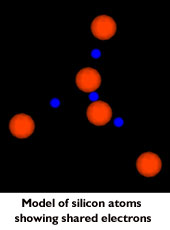
The
electrical properties of semiconductors change when they are compounded
with other elements during the manufacturing process. This process
is known as doping. In the manufacture of semiconductor devices,
additional elements are added to pure silicon in order to change
the electrical properties of the semiconductor and control the flow
of electrons through it. Diodes, transistors, resistors and capacitors
can all be created within a single chip of silicon or germanium
by precise control of the pattern and intensity of doping.
NEXT

|

