|
|
SemiconductorsPart 1: Atomic StructureThe Bohr Model
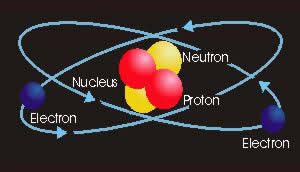
In the early twentieth century, scientists began to uncover the secrets of the atom. They found that the atom consisted of a nucleus, containing neutrons and protons, around which orbit electrons. These high energy particles are held together by the electrical attraction between the positive protons and the negative electrons. This description of the atomic structure is known as the Bohr model. Later research showed that the protons, neutrons and electrons are in fact made of up even smaller particles. For more information on quantum physics, visit the web sites of these major research facilities: Fermi National Accelerator Laboratory in the United States and CERN in Switzerland. Releasing the tremendous power locked in the atom can create nuclear energy and atomic bombs. Knowledge of the atomic structure also enabled researchers to develop the transistor. Normally there are an equal number of electrons and protons in every atom, and the number of electron-proton pairs (the atomic number) determines the physical and electrical properties of the element. If an electrical force removes or adds electrons to the atom, it becomes a positive ion (missing electron) or a negative ion (extra electron). The electrons orbit the central nucleus at different energy levels known as orbital rings or shells.
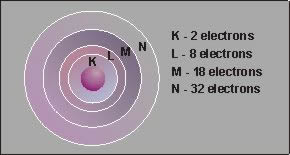
The diagram shows the maximum number of electrons which may be located in each shell of an atom. The outer shell, however, never contains more than 8 electrons. Elements with 8 electrons in the outer shell (or two, in the case of helium) are stable and do not interact chemically with other elements. These are the inert gases. The atomic structure also determines whether an element will be an electrical conductor, and insulator, or a semiconductor. |